- Pharmaceuticals
- Hypodermic Supplies
- IV Solutions
- IV Sets and Access Devices
- Exam Room Supplies
- Patient Treatment Supplies
- Oral Supplements
- Shop
- Learn
- Pharmaceuticals
- Hypodermic Supplies
- IV Solutions
- IV Sets and Access Devices
- Exam Room Supplies
- Patient Treatment Supplies
- Oral Supplements
- Shop
- Learn
- My Account
- Customer Service
- About
- Information
- Contact Us
-
Toll-Free: 800-854-7220
Hours: 7:00 AM to 5:30 PM Pacific
Fax: 714-540-5614
Email: answers@mcguff.com
Enter a search term.
If you can't find what you were looking for, please contact us using one of the methods below so we can better serve you.
Ascorbic Acid Injection - International Sales / Clinical Trials
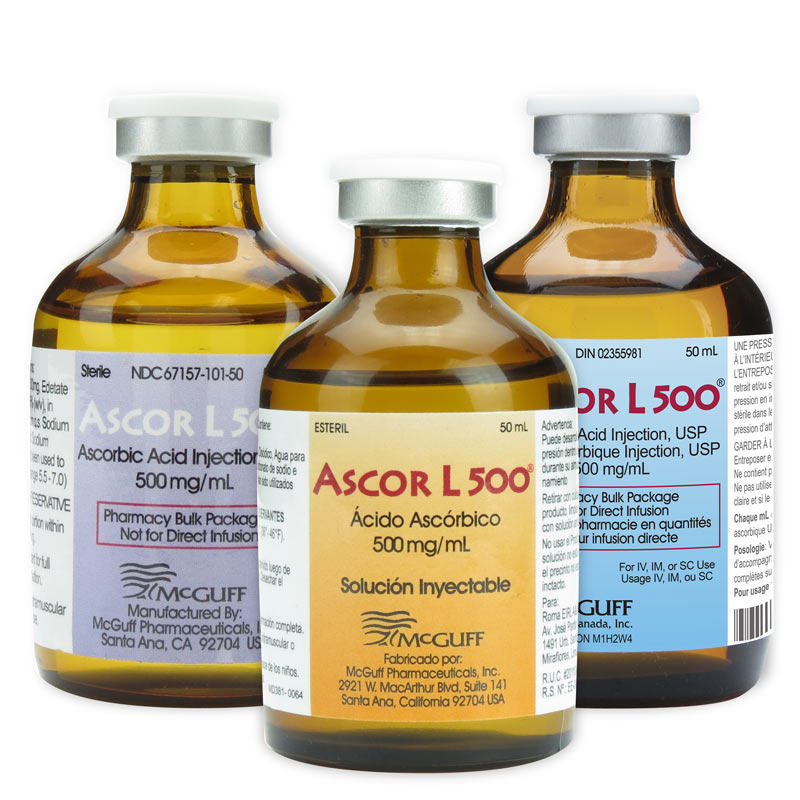
Ascorbic Acid Injection, USP 500mg/mL, PBP, 50mL Vial (International)
Manufactured by McGuff Pharmaceuticals, Inc (MPI). MPI is an FDA registered and inspected sterile fill pharmaceutical manufacturer located in the United States. This drug is approved for sale outside of the United States. Please contact our experienced customer service team.
Buy Internationally
Clinical Trials
McGuff is uniquely positioned to assist clinical trial investigators, directors and sponsors to perform Investigational New Drug (IND) clinical trial phases 1, 2, and 3.
We can provide customized clinical trial services through the McGuff Family of Companies based upon your specific needs.
- McGuff Pharmaceuticals, Inc., an FDA-reviewed manufacturer. McGuff Pharmaceuticals employ pharmacists, engineers, regulatory affairs, quality assurance and other personnel that have experience performing clinical trials.
- McGuff Compounding Pharmacy, Inc., a 503A registered compounding pharmacy specializing in unique formulations of sterile and non-sterile drugs. McGuff Compounding Pharmacy employs pharmacists, pharmacy techs, regulatory affairs, quality assurance and other personnel that have experience performing clinical trials.
We can provide you the information needed about the drug required for a smooth IND application.
Don't Miss Out!
Sign up to stay informed on McGuff news, insights, and promotions.Contact Us
- 1 (800) 854-7220
McGuff Company is LegitScript Certified
Copyright © 2024 McGuff Company, Inc. All rights reserved.
For information on the duties and responsibilities of this web site, please read our privacy policy and our warning and disclaimer. Use of this website signifies your agreement to the Privacy Policy and Warning Disclaimer.
