Enter a search term.
If you can't find what you were looking for, please contact us using one of the methods below so we can better serve you.
FAQ
minimum of 3 characters
No Search Results
Click on any of the questions below to reveal the answer:
Account Questions:
It's super simple, just follow the steps below. You will need to provide a:
- Bill to and Ship to address, telephone number and e-mail.
- Please submit a copy of your collaborating physician’s license if you do not have prescriptive authority/autonomous practice by your State Board.
- Copy of DEA Registration if controlled substances are ordered.
You can establish an account with McGuff by completing the login process on this website or by calling our customer service team at 1-800-854-7220. We are happy to help you with your account set up!
No, you don’t! All you will need is one website account and we will take care of the rest. We’ll process your order from the company you purchased from. Our goal is to make it as easy as possible to order your wholesale and compounded drugs and products!
A medical license with prescriptive authority is required for all Rx Products.
All McGuff customer service representatives are friendly and knowledgeable about all our product offerings. They are trained to handle all account types. However, we currently do not have regional or local account representatives.
Great question! There are three primary options to change your address:
- When you are in the process of placing a new order, you can change your address at that time. After placing the order with a changed address, a separate verification email will be sent to the email address on file to confirm changes were made to the account. Once that is confirmed by you, we will change your address on your account.
- Email or fax a signed letter from the provider on file specifying either an add-on address or an address change. You can do this at any time.
- Email or fax a voided prescription with the new address from the doctor on the account. You can do this at any time.
Please email the letter or voided prescription to answers@mcguff.com or feel free to fax it to us at (714) 540-5614.
We accept all major credit cards.
- Visa
- MasterCard
- American Express
- Discover
You may also sign up for Net 30 terms (application approval required once you have developed history).
To request a credit application form, email us at answers@mcguff.com or give us a call at 1-800-854-7220.
Applications may take a few weeks to be approved or denied. In the meantime, we recommend you make credit card payments to ensure you get your products in a timely manner. Also, we recommend doing this to establish a payment history with us.
Most of our products are for licensed providers with prescriptive authority, but we do offer many over-the-counter vitamins and supplies that do not require a medical license. Click the links below to view our options!
Generally, we are able to verify your account on the same day as long as everything is in good standing with the state and federal side. If we need additional information, we will reach out to you through your contact information.
Once we receive your order, we will verify your account license and shipping address quickly. Generally, we are able to verify a new account the same day and will ship out your order right away. We can even process it on the phone for you! If you have any questions about your account, do not hesitate to reach out.
Please remember, you do not need to wait to have your account verified before you place your first order.
Order Questions:
McGuff Medical: can ship to all 50 states, protectorates and internationally.
McGuff Outsourcing Solutions: you can find a list of all states and protectorates we ship to here.
With an ever-changing regulatory environment, we are doing our best to be licensed in all 50 states and protectorates, but it has become increasingly challenging. If you have any additional questions about where we ship, please contact us at at 1-800-854-7220.
Unfortunately, we can’t ship your orders together. McGuff Medical Products and McGuff Outsourcing Solutions are located in two separate locations, and state and federal laws do not allow us to mix products in the same package.
Please give us a call and speak with one of our customer service representatives. Please be advised, however, that orders are processed shortly after they are placed to ensure it ships out the same day.
We do run special discounts from time to time, such as conference attendees and special discounts from our customer service team. These discount codes can be applied at checkout. However, they can not be used for an already discounted product. These discounts are also can't be applied to ASCOR® orders.
Your account is only charged when an order has shipped from our facility. A pre-authorization is placed when the order is processed. This also applies to back-ordered products.
Good question. Here are some ways to find your tracking details:
With each online order you will receive a confirmation of shipment via email which will include your tracking information.
You can also track your order on your order history page here or check the status bar.
Yes, we do! We have a team of international advisors waiting to hear from you! Please contact us at (714)-545-2491 Monday - Friday, between 7:00 a.m. to 5:30 p.m. Pacific Time and let us know where you are located, what products you are interested in and any special requests. Click here for any other questions about international orders.
McGuff Compounded Drug Questions:
Great question! McGuff compounded drugs are made from our FDA-licensed McGuff Pharmaceuticals, Inc. manufacturing facility under the FDA and state requirements of a 503B Outsourcing Facility which allows us to manufacture drug products for distribution without formal drug approval from the FDA.
There are many differentiators, but it all stems from three main pillars:
- Quality: High quality, responsibly sourced, and consistent product. Without that, we believe that does not constitute a viable product. When you use our products, you can be confident knowing it is backed by our McGuff Quality Guarantee.
- Service: Without our customers (physicians, nurses and patients) we would not exist as a company. Since our inception, our excellent customer service has been at the forefront of what makes us stand out as a leader in the industry. We do everything in our power to ensure you have a positive experience when working with us.
- Involvement: We are not just another company simply offering products at a particular price. We are deeply involved with the market, working with both federal, state, international, and other agencies working to ensure necessary drugs are available to patients when they need them.
McGuff has a long and successful track record of successfully working with these agents to ensure continued patient access to these essential medications. A portion of the profits from these companies directly goes back to funding these endeavors.
Definitely! We have pharmacists on staff who would be more than happy to answer any questions you have. Contact us at 1-800-854-7220.
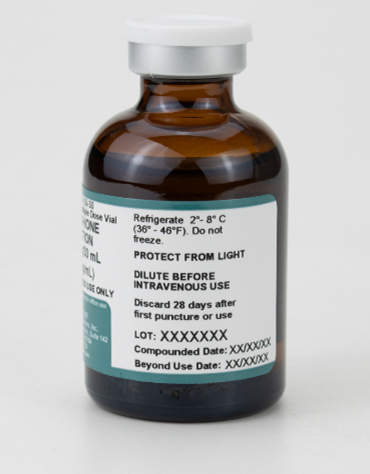
Each of our compounded drugs is completely unique. We perform in-depth stability studies of our products to ensure we assign accurate BUD. You can read the general BUD dating of each of our compounded products on each of their product pages under “Specific Attributes”. An example of which can be seen below:
Or you Contact Us and we will be happy to let you know.
Please contact us at 877-444-1133 to report an adverse event with one of our compounded drugs.
Product Questions:
A preservative, used in sterile injections, is an antimicrobial agent used to extend the shelf-life of the drug by retarding or reducing microbial proliferation.
Once the stopper of a preservative-free vial has been penetrated, you have two hours until it should be discarded. If unopened and properly stored, potency and sterility should be maintained until the expiration date on the vial label.
Learn more about what allocations are and what you can do to gain access to allocated IV solutions here.
Aluminum is omnipresent in nature, comprising 8% of the earth’s crust. Aluminum is present in soil, water, plants and animals. It is not feasible to eliminate aluminum completely from pharmaceutical raw materials. Consequently, you should assume that all injectable products contain aluminum. Disclosure of aluminum content on vials is not mandated except for those products that may be used in Total Parenteral Nutrition that could be administered to neonates.
A BD Vacutainer® Blood Collection Set is used for blood collection for laboratory analysis and a winged infusion set is used for administration of IV infusion therapy.
Any device that can connect to the internet with a modern browser will be able to access the software on your McGuff account.
While most of the vaccines we sell are thimerosal-free, a few vaccines still contain a trace amount of thimerosal. Thimerosal is still used in the early stages of manufacturing of a few vaccines to ensure the production line is sterile, but is removed through a purification process, with only trace amounts remaining. If you have any questions as to whether a vaccine is thimerosal-free or contains trace amounts of thimerosal, please email or call us.
If the product you're interested in is in stock, your customer service representative will be able to tell you the expiration date of the current lot being shipped. On average, manufactured products will have a year to a year and a half of useable shelf life before their expiration date.
Policies and Terms:
No, however, we do have an order processing and handling fee of $30 if your order is less than $50. This fee is waived if the order is over $50.
- 3-day shipping is FREE!
- 2-day shipping - $20 per package
- 1-day shipping - $35 per package
- Saturday Delivery Charge – additional $16 per package (for order placed on a Friday for overnight Saturday delivery)
- Non-Contiguous states require 2-Day shipping.
- Non-Contiguous United States – additional $30 per package (These states and protectorates include, but are not limited to: Alaska, Hawaii, Puerto Rico, US Virgin Islands, Guam, American Samoa, Northern Mariana Islands).
We do our very best to pack your product safely in as few of boxes as possible to ensure the lowest price to our customers. For Next Day or 2nd Day Air options, your final shipping cost will be determined at packing and invoicing, based on the number of boxes and the size and weight of products ordered, not the box size or destination.
Please note: compounded drugs will be shipped from McGuff Outsourcing Solutions in separate packaging.
If for any reason a product does not meet your expectations, we will, at our expense, pick up and return all unused products and provide you with a full refund or exchange (freight charges will not be refunded). All returns must be arranged within 30 days of receipt. Specially ordered items and refrigerated products may not be returnable. These items will be labeled with the following badges:

To initiate a return, contact us at 1-800-854-7220. Please have any identifying account information ready and we will be happy to assist you!
