Free Shipping on all orders over $50
Everything you need in one place!
Make a Selection
- Pharmaceuticals
- Hypodermic Supplies
- IV Solutions
- IV Sets and Access Devices
- Exam Room Supplies
- Patient Treatment Supplies
- Oral Supplements
- Shop
- Learn
Menu
- Pharmaceuticals
- Hypodermic Supplies
- IV Solutions
- IV Sets and Access Devices
- Exam Room Supplies
- Patient Treatment Supplies
- Oral Supplements
- Shop
- Learn
- My Account
- Customer Service
- About
- Information
- Contact Us
-
Toll-Free: 800-854-7220
Hours: 7:00 AM to 5:30 PM Pacific
Fax: 714-540-5614
Email: answers@mcguff.com
0
You have no items in your shopping cart.
Enter a search term.
If you can't find what you were looking for, please contact us using one of the methods below so we can better serve you.
1 (800) 854-7220
answers@mcguff.com
Suggest an Item
- Home /
- All Products /
- Pharmaceuticals /
- Injections /
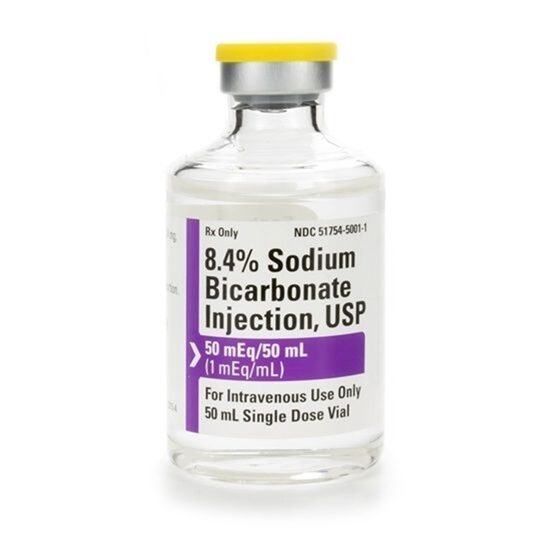
- Sodium Bicarbonate 8.4%, 50mEq/vial, SDV, 50mL Vial
Don't Miss Out!
Sign up to stay informed on McGuff news, insights, and promotions.
Wait...
Contact Us
- 1 (800) 854-7220
McGuff Company is LegitScript Certified
Copyright © 2024 McGuff Company, Inc. All rights reserved.
For information on the duties and responsibilities of this web site, please read our privacy policy and our warning and disclaimer. Use of this website signifies your agreement to the Privacy Policy and Warning Disclaimer.